Cβ-Selective Aldol Addition of D‑Threonine Aldolase by Spatial Constraint of Aldehyde Binding
American Chemical Society
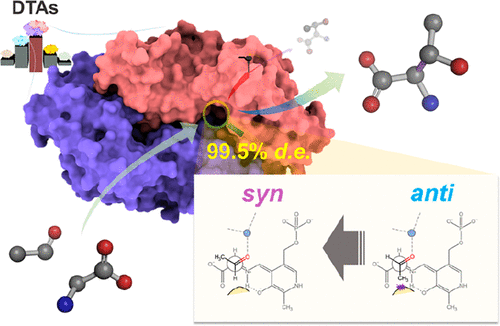
d-Threonine aldolase (DTA) is a useful biocatalyst that reversibly converts glycine and aldehyde to β-hydroxy-α-d-amino acid. However, low activity and poor diastereoselectivity limit its applications. Here we report DTA from Filomicrobium marinum (FmDTA) that shows much higher activity and Cβ-stereoselectivity in d-threonine production compared with those of other known DTAs. We determine the FmDTA structure at a 2.2 Å resolution and propose a DTA catalytic mechanism with a kernel of the Lys49 inner proton sink and metal ion in the aldol reaction cycle. The enzyme is rationally engineered to have high Cβ-stereoselectivity based on spatial constraint at the anti-specific aldehyde position in the mechanism, and the rational strategy is further applied to other DTAs for syn-production. The final FmDTAG179A/S312A variant exhibits a near-perfect 99.5% de value for d-threonine and maintains the de value above 93% even under kinetically unfavorable conditions. This study demonstrates how a detailed understanding of the reaction mechanism can be used for rational protein engineering.
DOI:10.1093/nar/gkac206. IF.(y). Citation . ISSN no.6892-6899.